Chemistry of a Plasma Ball
Hopewell Valley Student Podcasting Network
Chemistry Connections
Chemistry of a Plasma Ball
Episode #14
Welcome to Chemistry Connections, my name is Daniel Wolf and I am your host for episode #14 called Chemistry of a Plasma Ball. Today I will be discussing plasma, electron transitions, ionization energy, and noble gases.
Segment 1: Introduction to The Plasma Ball
In this segment, I want to briefly overview what a plasma ball is and where it came from. Nikola Tesla, a famed scientist for his many breakthroughs in electricity, invented and patented the “plasma lamp” while experimenting with high voltage phenomena. In 1971, another scientist named Bill Parker would invent the modern version of the plasma ball. James Falk would later commercialize it as a novelty toy.
How it works:
- A high voltage alternating current is emitted from the small electrode in the center of the plasma globe
- The globe itself contains a mixture of inert noble gases in a vacuum-sealed container
- The high voltage alternating current ionizes the gas creating plasma, and an electric current is allowed to flow.
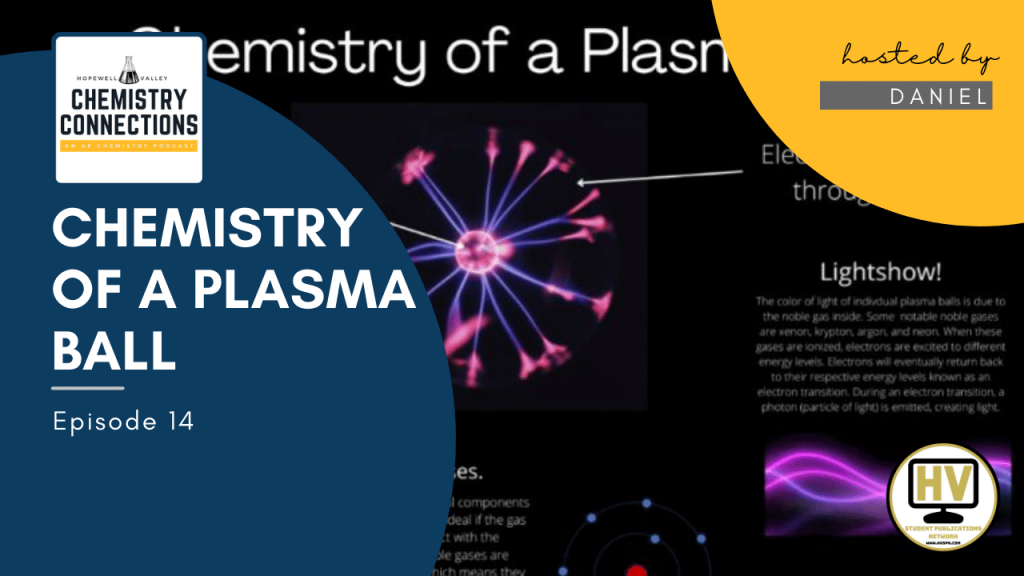
- Plasma filaments extend from the coil- the lightning effect seen extending from the coil
- The color of the light is dependent on the noble gas being ionized in the plasma ball
- The flow of electrons and the noble gas involved creates plasma filaments that radiates across the globe
- A human is much more conductive than glass, which is why the plasma filaments become a large singular beam, because it’s looking for a “ground”
Segment 2: The Chemistry Behind the Plasma Ball
There are quite a few connections to chemistry within a plasma ball. For example, the fact that plasma balls contain the fourth state of matter plasma.
- Simplified, when a solid is heated it turns into a liquid, when a liquid is heated it turns into a gas, and when a gas is heated it becomes plasma.
- It takes around 10,000 K – 100,000 K to create plasma (10-100 electron volts (eV))
- Ionization energy is the energy required to remove a single electron from an atom. Plasma is formed when electrons from gas are ionized, creating a soup of electrons and positive ions.
- Electricity (a flow of electrons) collides with noble gas atoms in the plasma ball. Electrons attached to the atoms are knocked off. Standard plasma balls contain 2-5 kilowatts of electricity at 30Hz.
- Lightning can be seen in the plasma ball due to the properties of plasma, being that it can conduct electricity due to the free-flowing charged particles (cations and electrons). Electrons are held together by electrostatic attractions
- Comparing the other states of matter, solids tend to have very packed and tight-fitted particles in a lattice structure. It’s classified by its definite shape and volume.
- Liquids have particles that move and slide past each other. There is more freedom in a liquid’s movement, so it has an indefinite shape and volume
- Down to the atomic structure, gases tend to have particles that move with higher speed and kinetic energy, there is a great amount of space between particles making the particles much more dispersed. Indefinite shape and volume
What about the different colors of plasma ball lightning. Some plasma balls emit a green color, while others emit a purple color.
- Electricity excites the electrons in the noble gas to different orbitals, when these electrons return back to their original orbitals in what’s called an electron transition, a photon is emitted.
- A photon is a particle of light and can be treated as such. Essentially the electron transition emits light. The color of light is dependent on the energy difference between two energy levels
- Example: an electron transition from the 3rd to the 1st energy level has a greater energy difference than an electron transition from the 2nd to 1st energy level.
- Example: neon causes a reddish-orange streamers while a mixture of neon, xenon, and krypton produces green streamers.
Why noble gases? After all, plasma can be created from any gas as long as it’s ionized.
- Noble gases are considered inert, which means they tend to be very nonreactive
- This is because of the full octet that all noble gases share. This means that no more electrons can be added to a noble gas.
- Components of plasma ball are mostly metal, so it would be good if the gas inside didn’t react
Segment 3: Personal Connections
I wanted to do this topic because I thought, when I was young, that plasma balls were one of the coolest toys back then, besides a power rangers action figure. For a state of matter that makes up 99.9% of the universe, we don’t see a lot of it on earth. So plasma balls gives us a glimpse into the wonders of plasma.
Thank you for listening to this episode of Chemistry Connections. For more student-ran podcasts and digital content, make sure that you visit www.hvspn.com.
Sources:
- https://wonderopolis.org/wonder/how-does-a-plasma-ball-work
- https://science.nasa.gov/science-news/science-at-nasa/1999/ast07sep99_1#:~:text=%2299.9%20percent%20of%20the%20Universe,NASA’s%20Marshall%20Space%20Flight%20Center.
- https://cen.acs.org/articles/86/i43/Plasma-Globes.html#:~:text=Plasma%20ball%20makers%20rely%20on,produces%20reddish%20orange%20light%20streamers.
- https://www.psfc.mit.edu/vision/what_is_plasma
- https://www.thenakedscientists.com/forum/index.php?topic=75251.0#:~:text=When%20electrons%20are%20split%20off,attached%20to%20a%20particular%20%2B%20ion.
- https://wonders.physics.wisc.edu/plasma-ball-experiments/#:~:text=The%20plasma%20ball%20is%20a,globe%20is%20a%20partial%20vacuum.
- https://sciencestruck.com/what-is-plasma-ball-how-does-it-work#:~:text=The%20first%20plasma%20lamp%20was,plasma%20ball%20in%20the%201970s.
- https://www.plasma-universe.com/plasma-filaments/
- https://www.advancedplasmasolutions.com/what-is-plasma/#:~:text=In%20thermal%20plasmas%2C%20energy%20is,100%20electron%20volts%20(eV)).
Music Credits
Warm Nights by @LakeyInspired

