Chemistry of Fireworks
Chemistry Connections
Episode #6
Welcome to Chemistry Connections, my name is Kristen McDonough and I am your host for episode #6 called the chemistry of Fireworks. Today I will be discussing what a firework is composed of and how they give us a colorful display in the air.
Segment 1: Introduction to Fireworks
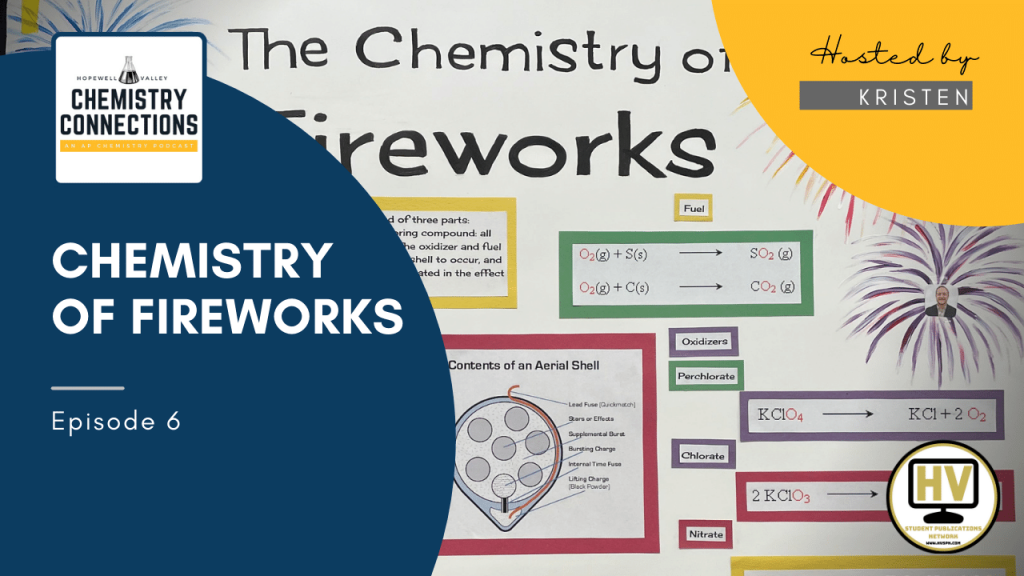
What is a firework made of?
Fireworks are composed of 3 different components; oxidizers, fuel, and color. The components of a firework are located in an aerial shell. The shell is launched into the air with black powder. Time fuse located inside the shell which causes the explosion of the shell in the air to be delayed. Effect pellets located inside the shell determine the characteristics of the firework. Color is determined by how different elements react with the heat from the explosion.
Segment 2: The Chemistry Behind Fireworks
Oxidizers are oxygen rich salts including potassium nitrate/perchlorate and strontium nitrate.
Nitrates (NO3-) are used for the initial upwards thrust. Not all of the oxygen gas is released which results in a slower combustion. The most common nitrate is potassium nitrate, which decomposes to potassium oxide, nitrogen gas, and oxygen gas. Chlorates (ClO3- ions) release all of the oxygen atoms in the form of oxygen gas but are highly unstable and are not commonly used in fireworks. Perchlorates (ClO4-) are often used instead. They release all of the oxygen atoms in the form of gas but are more stable. Lewis dot structure reveals that chlorates have a lone pair electron bonded to the central atom, whereas perchlorates do not, explaining the difference in stability.
The oxygen gas goes through a combination reaction with reducing agents such as sulfur and carbon otherwise known as the fuel. The fuel is a source of electrons, and in the reaction of oxygen gas and sulfur, sulfur dioxide is produced. The reaction is exothermic due to the greater energy released when the covalent bonds of the products are formed, resulting in the release of gas and heat causing the firework to explode
The color of the fireworks are determined by the metal cations in the salts in the effect pellets. Copper oxide produces blue, Strontium chloride produces red, Sodium silicate produces yellow, Calcium carbonate or nitrate produces orange, Barium acetate produces green.
Salts are used because they are easier to disperse and they’re less reactive compared to metals.
The different metals have different amounts of electrons in their outer shell. When they react with energy in the form of heat, the electrons jump from the ground state to the excited state.
The electrons release energy in the form of light when returning from the excited state to ground state, and the amount of energy they release determines the color. High energy released results in short wavelengths and a more blue violet color, whereas low energy released results in longer wavelengths and amore red orange color.
Segment 3: Personal Connections
Every fourth of July my family and I watch a fireworks display on the beach and we always have a lot of fun. Most people love the joy that fireworks give, so learning the chemistry behind fireworks has allowed me to connect my favorite holiday to chemistry.
Thank you for listening to this episode of Chemistry Connections. For more student-ran podcasts and digital content, make sure that you visit www.hvspn.com.
Sources:
https://www.youtube.com/watch?v=nPHegSulI_M
https://www.youtube.com/watch?v=qnA-rH1jwKA
http://www.scifun.org/CHEMWEEK/fireworks/Fireworks2017.htm
Music Credits
Warm Nights by @LakeyInspired

